DONJOY 11-2161-9 X-Act ROM Lite User Guide
- June 6, 2024
- Donjoy
Table of Contents

DONJOY 11-2161-9 X-Act ROM Lite
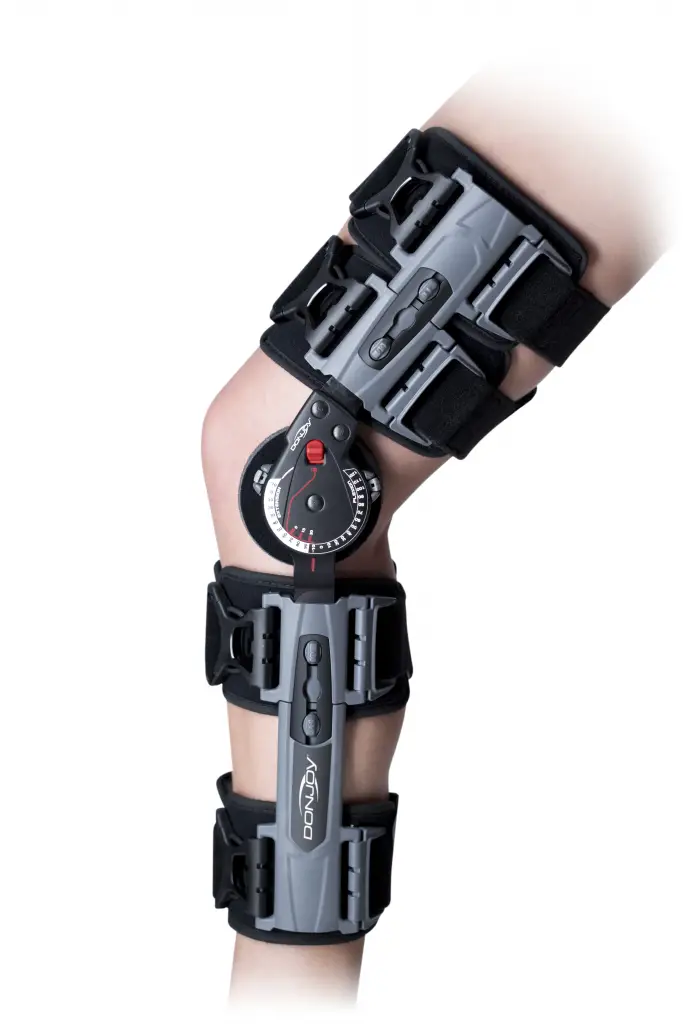 BEFORE USING THE DEVICE, PLEASE READ THE FOLLOWING INSTRUCTIONS
COMPLETELY AND CAREFULLY. CORRECT APPLICATION IS VITAL TO THE PROPER
FUNCTIONING OF THE DEVICE.
BEFORE USING THE DEVICE, PLEASE READ THE FOLLOWING INSTRUCTIONS
COMPLETELY AND CAREFULLY. CORRECT APPLICATION IS VITAL TO THE PROPER
FUNCTIONING OF THE DEVICE.
INTENDED USER PROFILE: The intended user should be a licensed medical professional, the patient or the patient’s caregiver. The user should be able to read, understand and be physically capable to perform all the directions, warnings and cautions provided in the information for use.
INTENDED USE/INDICATIONS: The DonJoy X-ACT ROM Knee Brace is designed to aid in immobilization of the knee and provide protected range of motion following ACL, PCL, LCL and MCL surgeries and meniscal repairs. Providing immobilization or controlled movement of a limb or body segment. Providing mild protection to a limb or body segment for acute and prophylactic care.
CONTRAINDICATIONS: Do not use if you are allergic to any of the materials contained in this product.
WARNINGS AND PRECAUTIONS:
- Loss of circulation, patient discomfort and patient re-injury are potential effects caused by device failure.
- ROM settings should not be changed without supervision of a medical professional.
- This product must be prescribed and fitted by a healthcare professional.
- The frequency, duration of use and directions for use should be determined by your healthcare professional.
- If pain, swelling, changes in sensation or other unusual reactions occur while using this product, you should contact your doctor immediately.
- The support should be snug but not impair circulation.
- Do not use over open wounds.
- Do not use the device if it is damaged and/or the packaging has been opened.
NOTE: Contact manufacturer and competent authority in case of a serious incident arising due to usage of this device. APPLICATION
INFORMATION:
-
Unfasten strap ends near black buckles and open brace flat by pulling both uprights apart.
-
Place leg on top of flat brace. Shorter upright is applied to the thigh. Adjust brace until each upright is aligned to centerline of the medial and lateral sides of the leg.
-
Telescope paddles to desired length by depressing the slider button until the desired length is reached. Ensure that both bars are equal in length and that each strap paddle is indexed to same position on each upright respectively.
-
Adjust the strap lengths:
- Unfasten strap from upright
- By pulling strap away from the body, remove slack from the backside of brace
- Re-fasten strap to upright to maintain length
- Ensure uprights are aligned on medial and lateral sides of leg
-
Clip each of the black buckle clips to the slider until a audible “click” is achieved. Tighten straps by pulling on loose end. Remove excess strap and reposition alligator strap end to new edge of the strap. Secure strap-end to strap.
-
A) Adjust the hinge by depressing the Flexion and Extension buttons inward and rotate stop until the desired angle aligns with button center. Release push button and ensure it is fully retracted to the extend position.
B) Utilize the quick-lock feature at 0, 15, or 30 degrees, by sliding the red quick lock button inward until the lock is engaged. Ensure uprights do not rotate. If another lock setting is required, see step 7 and ensure both Flexion and Extension buttons are set at the same angle setting. -
To remove brace, release each buckle. Brace can then be re-applied as a single unit.
OTHER ADJUSTMENTS:
The hinge bars may be bent to add varus or valgus contouring. Bend each bar by holding thigh/calf bar firmly against a solid surface and apply gentle and constant pressure to the hinge in the direction desired. Bend each side bar an equal amount above and below the hinge. For optimal ease of application post- operatively, pre-fit the brace prior to surgery if possible.
CLEANING INSTRUCTIONS:
Hand wash foam liners in water (30°C) with mild detergent. Rinse thoroughly.
AIR DRY only, do not heat dry.
Regular cleaning of the brace is recommended.
WARRANTY:
DJO, LLC will repair or replace all or part of the unit and its accessories for material or workmanship defects for a period of six months from the date of sale.
COMPOSITION:
Polypropylene 30%, Aluminum 40%, Nylon 25%, Delrin 3%, Stainless Steel 2%.
INTENDED FOR USE ON A SINGLE PATIENT.
NOT MADE WITH NATURAL RUBBER LATEX. RX ONLY.
NOTICE:
WHILE EVERY EFFORT HAS BEEN MADE IN STATE-OF-THE-ART TECHNIQUES TO OBTAIN THE MAXIMUM COMPATIBILITY OF FUNCTION, STRENGTH, DURABILITY AND COMFORT, THIS DEVICE IS ONLY ONE ELEMENT IN THE OVERALL TREATMENT PROGRAM ADMINISTERED BY A MEDICAL PROFESSIONAL. THERE IS NO GUARANTEE THAT INJURY WILL BE PREVENTED THROUGH THE USE OF THIS PRODUCT.
CAUTION:
FEDERAL LAW (U.S.A.) RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A LICENSED HEALTH CARE PROFESSIONAL.
Image Description







Read User Manual Online (PDF format)
Read User Manual Online (PDF format) >>